REFPROP - 制冷剂物性查询软件
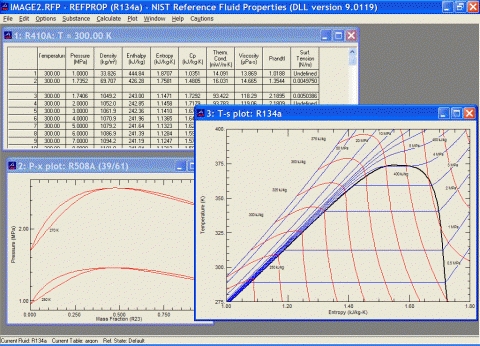
REFPROP是一款用于制冷剂物性查询的软件。它是一款工质物性计算软件,该软件由美国标准技术研究所(NIST)研制开发。从检索到的文献来看,REFPROP被很多研究项目用作物性数据源,或作为计算结果性的参考数据源。
REFPROP版本10的新功能
NIST REFPROP程序的大部分功能都已得到增强,图形界面,Excel电子表格,Fortran文件(即核心属性例程),C ++,MATLAB,VB等中的示例程序以及流体。下面列出了一些的改进:
-
提高计算速度。
-
新的功能,允许用户使用相同的命令调用Refprop来处理输入/输出属性,而学习TPFLSH,THERM等的输入/输出。
-
新的快捷命令来加载流体和混合物和方法来地使用代码。
-
Gernert混合模型用于选定的混合物与水,水+ CO2和湿空气。
-
Mac的新DLL;这允许使用Refprop,例如Excel 2011和2016的Mac版本。
-
氨,氦和重水的新的参考方程式。
-
加入下列流体:氯气,二氯乙烷,乙二醇,环氧乙烷,十六烷,二十二烷,R1233zd(E),R1243zf,R1336mzz(Z)和氯乙烯。
-
已经对环戊烷,D4,庚烷,己烷,氯化氢,硫化氢,异戊烷,MDM,MM,辛烷,戊烷,R161,R245fa,R32,RE347mcc(HFE-7000),二氧化硫和氙。
-
提高了氨/水和乙二醇/水的度的新混合物模型。
-
(R),R125,R134a和R1234ze(E),R1234ze(E)与R125和R134a以及CO2 / R1216的R1234yf配合(或改装)混合物参数
-
对二元混合物(正构烷烃+正构烷烃混合物,与二氧化碳的混合物)选定系列的新估算方案,以获得尚未拟合的混合物的估计相互作用参数。
-
亨利的恒定估计方案,以获得更好的VLE起始值的混合物。
-
Fortran代码都经过了高度,增加了新的注释来解释代码的工作。
-
识别用于确定相位的III型混合物的附加代码。
-
丙酮,苯,1-丁烯,二氧化碳,硫化羰,顺式丁烯,环己烷,环戊烷,环戊烷,D4,D5,D6,二氯乙烷,乙醚,碳酸二甲酯,二十二烷,乙烯,环己烷,异丙烷,异戊烷,间二甲苯,MD2M,MD3M,MD4M,MDM,MM, R114,R161,R1233zd(E),R1234yf,R1234ze(Z),R1243zf,R245fa,RE143a,RE347mcc,R40,反式丁烯,甲苯,十一烷和氯乙烯。
-
固定氢或氦混合物的传输中的缺陷。
-
纯液体的大多数表面张力方程已被更新。
-
用于混合物的新的表面张力模型的不确定性较低。
-
用于计算文丘里喷嘴形成热量和质量流量的新代码。
操作系统要求:
PC运行Windows ® XP,7,8,或10; 10.0 MB 可用硬盘空间
英文简介
New Features of REFPROP Version 10
-
Enhancements have been made to most areas of the NIST REFPROP program, including the equations of state for many of the pure fluids and mixtures, the transport equations, the graphical interface, the Excel spreadsheet, the Fortran files (i.e., core property routines), the sample programs in Python, C++, MATLAB, VB, etc. Some of the more important improvements are listed below:
-
A new Excel file with many more examples and additional documentation.
-
All of the Fortran code was highly optimized resulting in increased calculation speed and improved convergence. Many new flags were added to allow the user to specify better how the programs works.
-
A new function is available to allow users to call Refprop with one single command that replaces most other calls from 9.1 (thus removing the need to learn what routines to use and the inputs/outputs for each routine, such as TPFLSH, THERM, etc.) However, the old routines are still available for backwards compatibility.
-
New shortcut keywords to load fluids and mixtures and other methods to simplify use of the code.
-
New shared library for the Mac; this allows use of Refprop with, for example, Python or Excel 2011. A CMake‑based build system allows for compilation on any platform (windows, OSX, Linux).
-
The vapor‑liquid equilibrium calculations for tracing isotherms and isobars (T‑x and p‑x diagrams) are greatly improved (doi: https://doi.org/10.1002/aic.16074).
-
New reference equations of state for ammonia, helium, and heavy water. The ammonia equation of state introduces the first change to the Helmholtz energy functional form in over 25 years of development of equations for the thermodynamic properties of fluids.
-
The addition of the following refrigerants: R1123, R1224yd(Z), R1233zd(E), R1234ze(Z), R1243zf, and R1336mzz(Z).
-
The addition of the following fluids: 1,3‑butadiene, 1‑butyne, 1‑pentene, 2,2‑dimethylbutane, 2,3‑dimethylbutane, 3‑methylpentane, acetylene, chlorine, chlorobenzene, cyclobutene, 1,2‑dichloroethane, diethanolamine, docosane, ethylene glycol, ethylene oxide, hexadecane, monoethanolamine, perfluorohexane, propadiene, propylene oxide, and vinyl chloride.
-
New equations of state have been developed for cyclopentane, D4, heptane, hexane, hydrogen chloride, MDM, MD2M, MM, neon, octane, pentane, perfluorobutane, perfluoropentane, R‑1233zd(E), R‑161, R‑245fa, R‑E347mcc (HFE‑7000), and sulfur dioxide. The development of an equation of state is a complex process requiring many months of work for each one.
-
Mixture model of Gernert implemented for selected mixtures with water, including water+CO2 and moist air.
-
Transport equations have been added or modified for acetone, acetylene, ammonia, benzene, butane, 1,3‑butadiene, 1‑butene, 1‑butyne, 2,2‑dimethylbutane, 2,3‑dimethylbutane, carbon dioxide, carbon monoxide, carbonyl sulfide, chlorine, chlorobenzene, cis‑butene, cyclobutene, cyclohexane, cyclopentane, cyclopropane, D4, D5, D6, 1,2‑dichloroethane(R150), diethanolamine, diethyl ether, dimethyl carbonate, dimethyl ether, docosane, ethane, ethylbenzene, ethylene, ethylene glycol, ethylene oxide, fluorine, heptane, hexane, hexadecane, hydrogen chloride, hydrogen sulfide, isobutene, isohexane, isooctane, isopentane, krypton, methyl palmitate, methyl linolenate, methyl linoleate, methyl oleate, methyl stearate, m‑xylene, MD2M, MD3M, MD4M, MDM, MM, methylcyclohexane, 3‑methylpentane, monoethanolamine, neon, neopentane, nitrous oxide, Novec‑649, o‑xylene, p‑xylene, pentane,1‑pentene, propadiene, propylcyclohexane, propylene, propylene oxide, propyne, perfluorobutane, perfluoropentane, perfluorohexane, propane, R1123, R143a, R114, R161, R1224yd(Z), R1233zd(E), R1234yf, R1234ze(Z), R1234ze(E), R1243zf, R13I1 (CF3I), R1336mzz(Z), R218, R236fa, R236ea, R245ca, R245fa, R365mfc, RE143a, RE245cb2, RE245fa2,RE347mcc, RC318, R40, sulfur dioxide, trans‑butene, toluene, undecane, vinyl chloride, and xenon.
-
New mixture models for ammonia + water and ethylene glycol + water.
-
Approximately 400 binary pairs have been added from the work of Bell and Lemmon (doi: https://pubs.acs.org/doi/abs/10.1021/acs.jced.6b00257 ) • Mixture parameters were fitted (or refitted) for the following binary mixtures: R1234yf with R32, R125, R134a, and R1234ze(E), R1234ze(E) with R125 and R134a, and many others. These new mixing parameters with R1234yf and R1234ze(E) are currently the standard used in the refrigeration industry and Version 10 puts all users in compliance with the property values now in use world‑wide. All new ASHRAE predefined mixtures except those with trans‑1,2‑dichloroethylene (t‑EDC) (due to the lack of a pure fluid equation) are included.
-
New estimation schemes were developed for selected families of binary mixtures (n‑alkane + n‑alkane mixtures, mixtures with CO2, etc.) to obtain estimated interaction parameters for mixtures that have not been fitted.
-
A reverse Polish type notation was added to read any functional form for the transport properties, eliminating the need to compile a new DLL as new correlations are published. The notation and corresponding coefficients of the equation are simply added to the fluid files and the new code will read and interpret the supplied text.
-
The DOI for each primary equation was added to the fluid files. A link in the GUI is now available to load the publication if access to the journal is available.
-
Henry's constant estimation scheme to obtain better starting values for VLE of mixtures to improve convergence.
-
Additional code to identify type III mixtures for use in phase determination.
-
Most surface tension equations for the pure fluids have been updated, and an improved surface tension model for mixtures was added.
-
New code to calculate heat of formation or the mass flux for a Venturi nozzle.
Version 10.0 includes 147 pure fluids, 5 pseudo-pure fluids (such as air), and mixtures with up to 20 components:
-
The typical natural gas constituents methane, ethane, propane, butane, isobutane, pentane, isopentane, hexane, isohexane, 2,2-dimethylbutane, 2,3-dimethylbutane, 3-methylpentane, heptane, octane, isooctane, nonane, decane, undecane, dodecane, carbon dioxide, carbon monoxide, hydrogen, nitrogen, and water.
-
The hydrocarbons 1,3-butadiene, 1-butene, 1-butyne, 1-pentene, acetone, acetylene, benzene, butene, cis-butene, cyclobutene, cyclohexane, cyclopentane, cyclopropane, docosane, ethylene, hexadecane, isobutene, methylcyclohexane, neopentane, propadiene, propylcyclohexane, propyne, toluene, and trans-butene.
-
The HFCs R23, R32, R41, R125, R134a, R143a, R152a, R161, R227ea, R236ea, R236fa, R245ca, R245fa, R365mfc, R1123, R1224yd(Z), R1233zd(E), R1234yf, R1234ze(E), R1234ze(Z), R1243zf, and R1336mzz(Z).
-
The refrigerant ethers RE143a, RE245cb2, RE245fa2, and RE347mcc (HFE-7000).
-
The HCFCs R21, R22, R123, R124, R141b, and R142b.
-
The traditional CFCs R11, R12, R13, R113, R114, and R115.
-
The fluorocarbons R14, R116, R218, R1216, C4F10, C5F12, C6F14, and RC318.
-
The "natural" refrigerants ammonia, carbon dioxide, propane, isobutane, and propylene.
-
The main air constituents nitrogen, oxygen, and argon.
-
The noble elements helium, argon, neon, krypton, and xenon.
-
The cryogens argon, carbon monoxide, deuterium, krypton, neon, nitrogen trifluoride, nitrogen, fluorine, helium, methane, oxygen, normal hydrogen, parahydrogen, and orthohydrogen.
-
Water (as a pure fluid, or mixed with ammonia).
-
Ethylene glycol (as a pure fluid, or mixed with water).
-
The fluids carbonyl sulfide, chlorine, chlorobenzene, dichloroethane, diethanolamine, diethyl ether, dimethyl carbonate, dimethyl ether, ethanol, ethylene oxide, heavy water, hydrogen chloride, hydrogen sulfide, methanol, methyl chloride, monoethanolamine, nitrous oxide, Novec-649, propylene oxide, sulfur dioxide, sulfur hexafluoride, trifluoroiodomethane, and vinyl chloride.
-
The xylenes m-xylene, o-xylene, p-xylene, and ethylbenzene.
-
The FAMES (fatty acid methyl esters, i.e., biodiesel constituents) methyl oleate, methyl palmitate, methyl stearate, methyl linoleate, and methyl linolenate.
-
The siloxanes octamethylcyclotetrasiloxane, decamethylcyclopentasiloxane, dodecamethylcyclohexasiloxane, decamethyltetrasiloxane, dodecamethylpentasiloxane, tetradecamethylhexasiloxane, octamethyltrisiloxane, and hexamethyldisiloxane.
-
121 predefined mixtures (such as R407C, R410A, and air); the user may define and store others.
- 2026-02-09
- 2026-01-20
- 2026-01-16
- 2026-01-12
- 2026-01-12
- 2026-01-09
- 2026-03-09
- 2026-03-09
- 2026-03-09
- 2026-03-09
- 2026-02-26
- 2026-02-26
















